IAT-TOP
Intra-arterial Thrombolysis After Successful Thrombectomy for Acute Ischemic Stroke Due to Large Vessel Occlusion in the Posterior Circulation
ClinicalTrials Identifier:NCT05897554
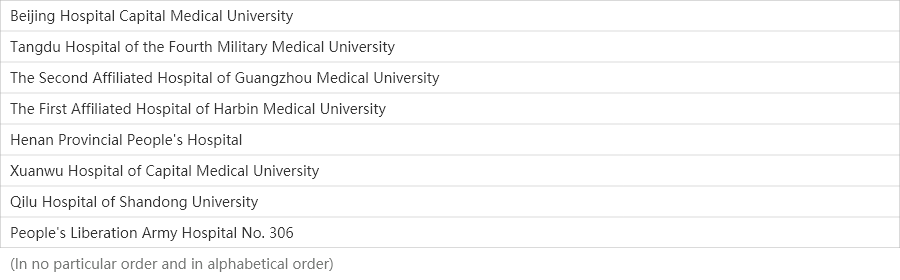
34
Medical centers
376
Patients
Ongoing
collection
10,000+
Images data
Introduction
In patients with acute posterior circulation large vessel occlusion, this multicenter randomized controlled trial aims to investigate the safety and effectiveness of intra-arterial alteplase in bridging arteries following successful recanalization by mechanical thrombolysis. A total of 34 Chinese tertiary hospitals will participate in this study, which is expected to enroll 376 patients with successful recanalization of acute posterior circulation ischemic stroke treated with EVT. These patients were randomized 1:1 into a trial group (intra-arterial alteplase bridging therapy) and a control group (no intra-arterial alteplase bridging therapy). All patients were required to undergo CT (CTA), MRI (MRA), and brain tissue angiography at baseline and on days 1, 2, 7, and 90 after enrollment. The database is expected to collect more than 1,000 cases and hundreds of thousands of image frames, which will be categorized, stored, and interpreted by specialized personnel.

Centers
CMOSS-2
Extracranial-intracranial Bypass Surgery Versus Medical Treatment Alone for Symptomatic Chronic Middle Cerebral Artery Occlusion
ClinicalTrials Identifier:NCT05899582
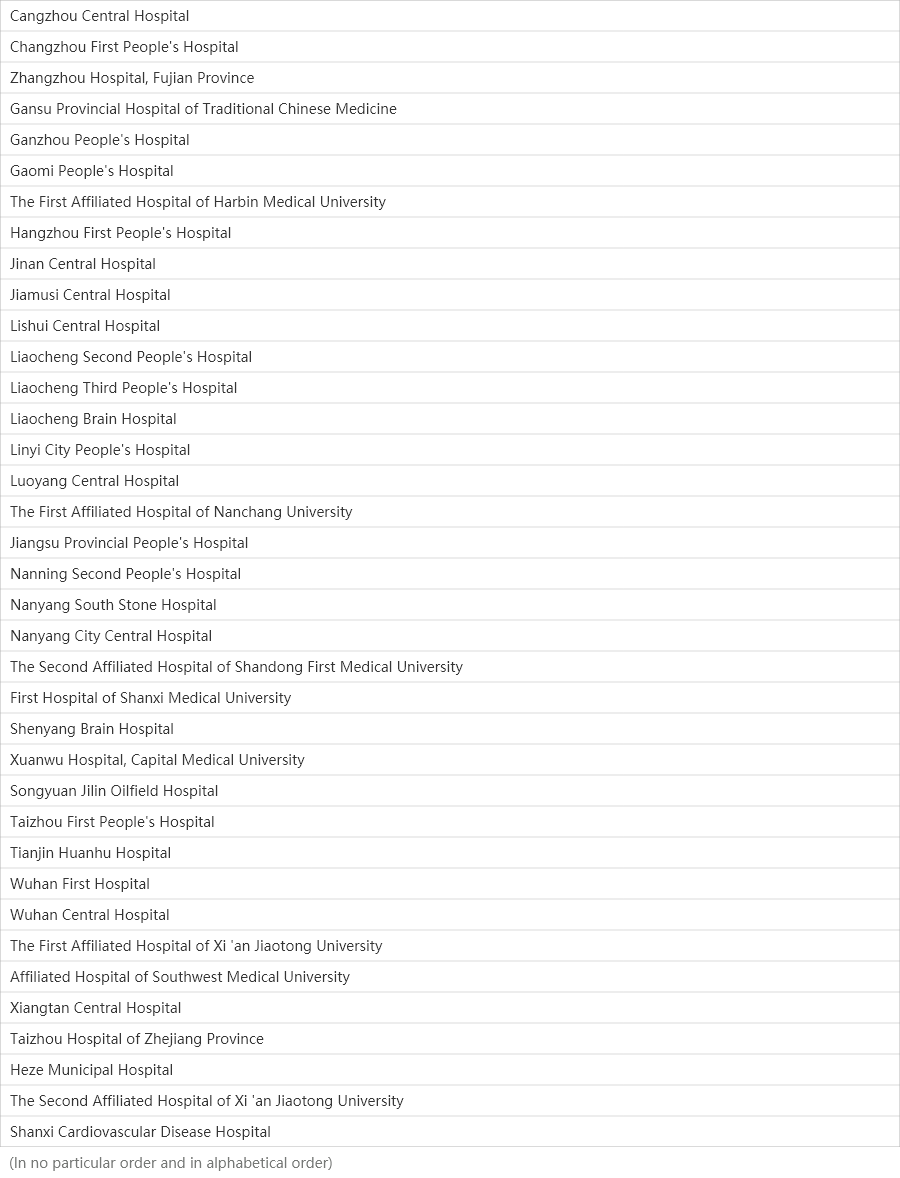
12
Medical centers
364
Patients
Ongoing
collection
10,000+
Images data
Introduction
This study is a phase II trial of the first multicenter randomized controlled trial of carotid or middle cerebral artery occlusion bypass grafting versus drug therapy in the field of neurointervention in China (CMOSS study). A total of 12 Chinese tertiary hospitals will participate in this study, which is expected to enroll 364 patients diagnosed with symptomatic unilateral chronic middle cerebral artery occlusion by total cerebral angiography. These patients were randomly divided 1:1 into a trial group (direct intracranial and extracranial vascular bypass surgery combined with standard drug therapy) and a control group (standard drug therapy). All patients will be required to undergo cerebral angiography, cerebral tissue perfusion, brain tissue, and cerebral vascular examinations at baseline, day 7, 30 days, 6 months, 12 months, and 24 months after enrollment. In this study, it is expected that more than one thousand cases of relevant imaging data and hundreds of thousands of image frames will be collected, and the relevant personnel will be respectively, categorized and organized, stored and interpreted.

Centers
VISTA
Drug-eluting Stenting Versus Medical Treatment Alone for Patients With Extracranial Vertebral Artery Stenosis(VISTA)
ClinicalTrials Identifier:NCT05885932
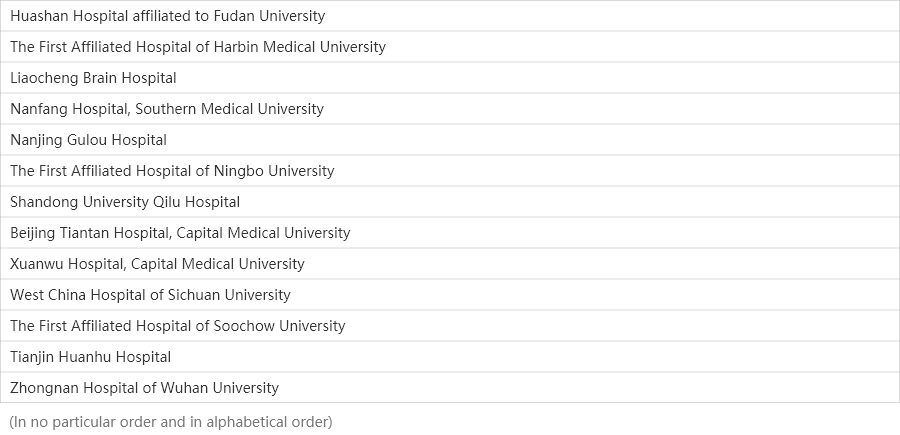
31
Medical centers
472
Patients
Ongoing
collection
10,000+
Images data
Introduction
This study is a multicenter randomized controlled trial comparing the efficacy and safety of rapamycin drug-eluting stents combined with drug therapy and drug therapy alone for symptomatic extracranial atherosclerotic stenosis. A total of 31 Chinese tertiary hospitals will participate in this study, which is expected to enroll 472 patients with severe stenosis of the symptomatic extracranial segment of the vertebral artery (V1-2) confirmed by total cerebral angiography. These patients were randomly divided 1:1 into a trial group (drug-eluting stent combined with optimal drug therapy) and a control group (optimal drug therapy). All patients were required to undergo cerebral angiography, brain tissue perfusion, brain tissue and cerebral vascular examination at baseline, 3 days, 30 days, 6 months, 12 months, 24 months and 36 months after enrollment. In this study, it is expected that more than one thousand cases of relevant imaging data and hundreds of thousands of image frames will be collected, which will be categorized, organized, stored, and interpreted by the relevant personnel, respectively.

Centers
CMOSS
Carotid and Middle cerebral artery Occlusion Surgery Study
ClinicalTrials Identifiers: NCT01758614
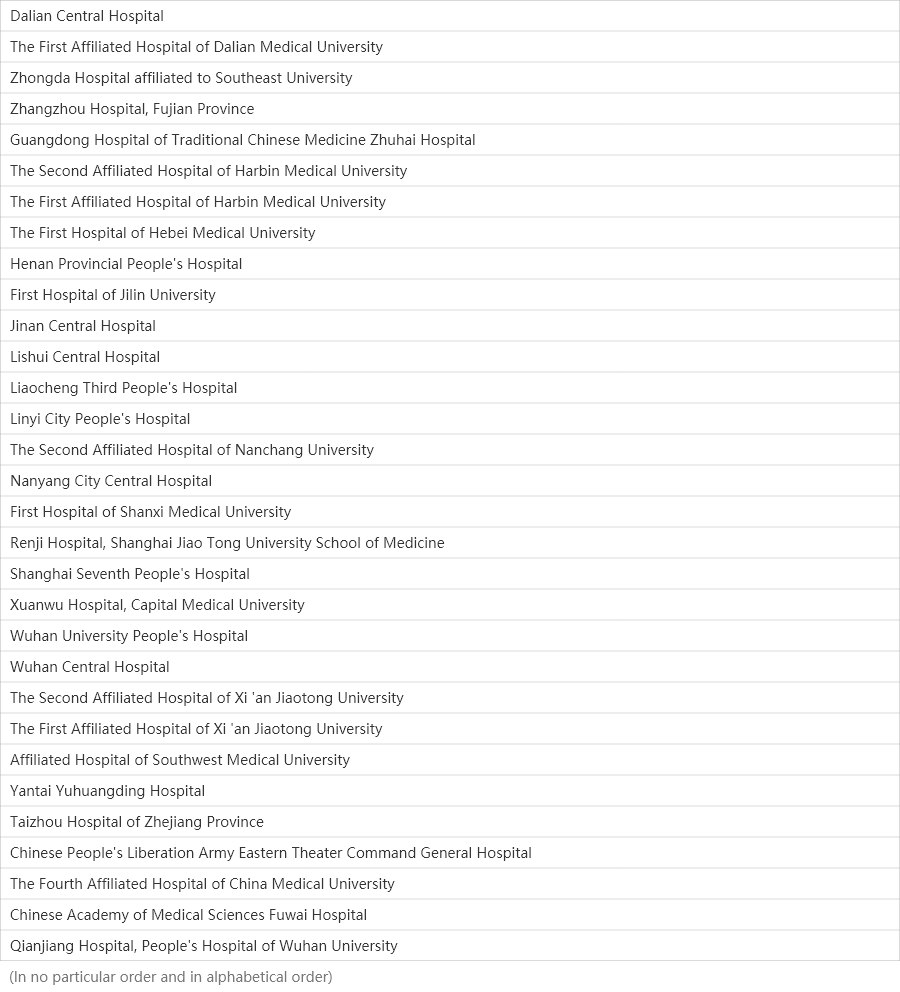
15
Medical centers
330
Patients
2100+
collection
100,000+
Images data
Introduction
CMOSS is the first multi-center randomized controlled trial on bypass surgery versus medical treatment for carotid or middle cerebral artery occlusion in China. A total of 15 medical centers were involved. 330 patients with symptomatic carotid or middle cerebral artery occlusion were included, which were randomly divided into the surgical treatment group and the medical treatment group at 1:1. All patients were required to undergo cerebral angiography, cerebral tissue perfusion, brain tissue, and cerebral vascular examination at baseline, 7th days, 30th days, 6th months, 12th months, 24th months after enrollment. A total of more than 2,100 images were collected, and hundreds of thousands of frames of image data were classified, sorted, stored, and interpreted.

Centers
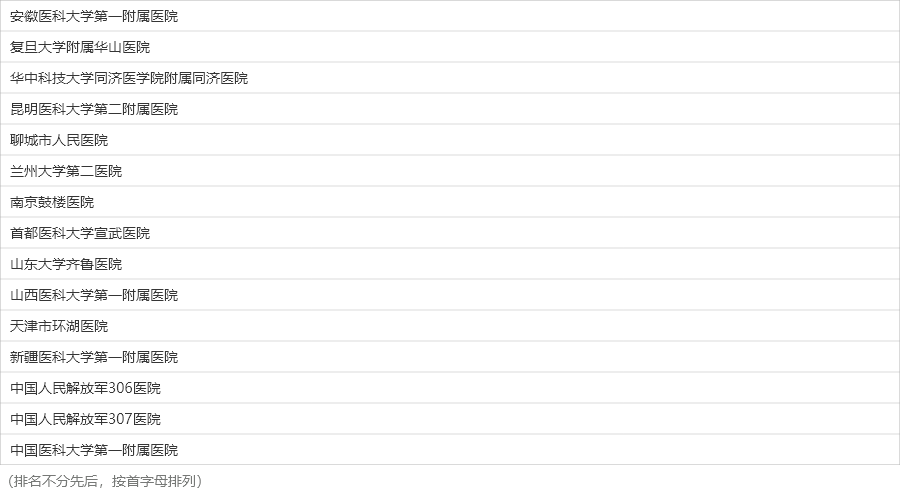
CASSISS
China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis
ClinicalTrials Identifiers: NCT01763320
8
Medical centers
380
Patients
2400+
Data collection
100,000+
Images data

Introduction
CASSISS is the first multi-center randomized controlled trial on endovascular treatment versus medical treatment for intracranial atherosclerotic stenosis in the neurointerventional community in China. A total of 8 medical centers were involved. 380 patients with symptomatic intracranial atherosclerotic stenosis more than 70% degree were included, which were randomly divided into the endovascular treatment group and the medical treatment group at 1:1. All patients were required to undergo vascular ultrasound, cerebral angiography, brain tissue perfusion, and brain tissue examination at baseline, 1st day, 7th days, 30th days, 4th months, 12th months, 24th months, and 36th months after enrollment. A total of more than 2,400 images were collected, and hundreds of thousands of frames of image data were classified, sorted, stored, and interpreted.
Centers